Bait And Switch

Bait and Switch Definition
A “bait and switch” takes place when a seller creates an appealing but ingenuine offer to sell a product or service, which the seller does not actually intend to sell. This initial advertised offer is “the bait.” Then the seller switches customers from buying the advertised product or service that the seller initially offered into buying a different product or service that is usually at a higher price or has some other advantageous effect to the advertiser. This is the “switch.” Normally, the switched product that the consumer buys is usually at a higher purchase price, an increased profit for the seller, or may have a less marketable characteristic than the product advertised.
Several weeks ago, it was heralded that the Pfizer jab which was manufactured under emergency use authorization was fully approved by the FDA for standard use. This was understood by the public and promoted as such by the government and media that the Pfizer Covid-19 vaccination is no longer an experimental vaccine but is now perfectly safe to use, given its now designated standard use authorization.
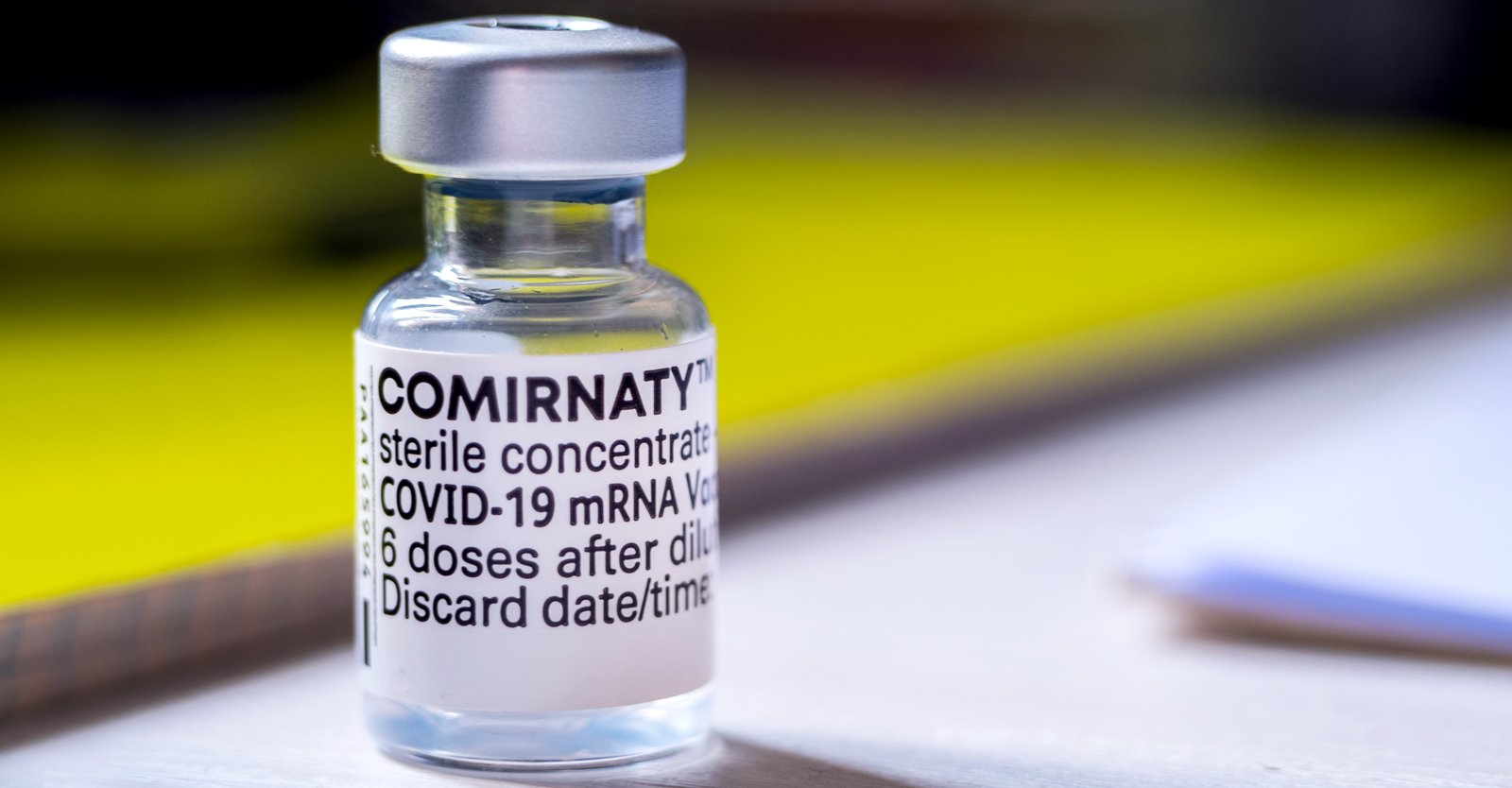
But as usual, the pharmaceutical giant and the FDA cannot be trusted as the devil is found in the details. It turns out that when one examines the details of this classic bait and switch scheme foisted upon the unknowing public, neither Pfizer nor the FDA bothered to reveal the actual identity of the vaccine product that was approved. The vaccine that the FDA approved for standard use is not the EUA Pfizer Covid-19 vaccine that has been administered throughout the United States. Rather, the FDA in August approved the Pfizer-BioNTech version of the vaccine named Comirnaty – which is distributed in Europe – not the United States.

Pfizer claims that Comirnaty and its EUA-authorized vaccine marketed in the USA are of the same formulation and the products can be used interchangeably to provide the vaccination series without presenting any safety or effectiveness concerns. However, U.S. Federal District Judge Allen Winsor of the U.S. District Court for the Northern District of Florida has ruled that the Comirnaty vaccine and Pfizer's EUA vaccine are not interchangeable due to the difference in ingredients and the manufacturing process.
A federal district court judge has rejected a claim by the U.S. Department of Defense (DOD) that the Pfizer-BioNTech COVID-19 vaccine being administered under Emergency Use Authorization is interchangeable with Pfizer’s Comirnaty vaccine, which in August was fully licensed by the U.S. Food and Drug Administration (FDA).
Under law, everyone has ‘right to refuse’ EUA product.
The Nuremberg Code and federal law state that no person can be forced to participate in medical experiments which include experimental vaccination. People in the USA who were vaccine-hesitant were led to believe that the Pfizer jab was no longer under experimental use status and proceeded to get their shots under the impression that it was fully approved. Other people were required to take the jab due to government and employer mandates as well as school students in order to continue being enrolled. The vaccination requirement is unlawful under EUA status but is nonetheless still being enforced. Suffice to say, the lack of transparency by the vaccine manufacturers and the FDA does nothing to engender the public's trust and confidence regarding vaccine safety. Buyer beware.





